 A US Food and Drug Administration (FDA) panel has opened a new era in medicine, unanimously recommending that the agency approve the first-ever treatment that genetically alters a patient’s own cells to fight cancer, transforming them into what scientists call “a living drug” that powerfully bolsters the immune system to shut down the disease, writes Denise Grady for The New York Times.
A US Food and Drug Administration (FDA) panel has opened a new era in medicine, unanimously recommending that the agency approve the first-ever treatment that genetically alters a patient’s own cells to fight cancer, transforming them into what scientists call “a living drug” that powerfully bolsters the immune system to shut down the disease, writes Denise Grady for The New York Times.
If the FDA accepts the recommendation, which is likely, the treatment will be the first gene therapy ever to reach the market. Others are expected: researchers and drug companies have been engaged in intense competition for decades to reach this milestone, the report points out.
"Novartis is now poised to be the first. Its treatment is for a type of leukaemia, and it is working on similar types of treatments in hundreds of patients for another form of the disease, as well as multiple myeloma and an aggressive brain tumor.
"To use the technique, a separate treatment must be created for each patient — their cells removed at an approved medical centre, frozen, shipped to a Novartis plant for thawing and processing, frozen again and shipped back to the treatment centre."
According to the report, a single dose of the resulting product has brought long remissions, and possibly cures, to scores of patients in studies who were facing death because every other treatment had failed. The panel recommended approving the treatment for B-cell acute lymphoblastic leukaemia that has resisted treatment, or relapsed, in children and young adults aged three to 25.
One of those patients, Emily Whitehead (12) and the first child ever given the altered cells, was at the meeting of the panel with her parents to advocate for approval of the drug that saved her life. In 2012, as a six-year-old, she was treated in a study at the Children’s Hospital of Philadelphia.
"Severe side effects — raging fever, crashing blood pressure, lung congestion — nearly killed her. But she emerged cancer free, and has remained so." Emily’s father, Tom Whitehead, told the panel when approved the treatment could potentially save thousands of children's lives worldwide.
The main evidence that Novartis presented to the FDA came from a study of 63 patients who received the treatment from April 2015 to August 2016. Fifty-two of them, or 82.5%, went into remission — a high rate for such a severe disease. Eleven others died.
“It’s a new world, an exciting therapy,” Dr Gwen Nichols, the chief medical officer of the Leukemia and Lymphoma Society, which paid for some of the research that led to the treatment, said in the report.
The next step, she said, will be to determine “what we can combine it with and is there a way to use it in the future to treat patients with less disease, so that the immune system is in better shape and really able to fight.” She added, “This is the beginning of something big.”
At the meeting, the panel of experts did not question the lifesaving potential of the treatment in hopeless cases, the article continues. "But they raised concerns about potentially life-threatening side effects — short-term worries about acute reactions like those Emily experienced, and longer-term worries about whether the infused cells could, years later, cause secondary cancers or other problems.
"Oncologists have learned how to treat the acute reactions, and so far, no long-term problems have been detected, but not enough time has passed to rule them out.
"Patients who receive the treatment will be entered in a registry and tracked for 15 years.
"Treatments involving live cells, known as 'biologics' are generally far more difficult to manufacture than standard drugs, and the panelists also expressed concerns about whether Novartis would be able to produce consistent treatments and maintain quality control as it scaled up its operation."
The treatment was developed by researchers at the University of Pennsylvania and licensed to Novartis.
Use will not be widespread at first because the disease is not common. It affects only 5,000 people a year, about 60% of them children and young adults. Most children are cured with standard treatments, but in 15% of cases — like Emily’s and Connor’s — the disease does not respond, or it relapses.
Analysts predict that these individualised treatments could cost more than $300,000, but a spokesperson for Novartis, Julie Masow, declined to specify a price.
Although the figure may seem high, people with cancer often endure years of expensive treatment and repeat hospital stays that can ultimately cost even more.
Because the treatment is complex and patients need expert care to manage the side effects, Novartis will initially limit its use to 30 or 35 medical centers where employees will be trained and approved to administer it, the company said.
As to whether the treatment, known as CTL019 or tisagenlecleucel, will be available in other countries, is quoted in the report as saying: “Should CTL019 receive approval in the US, it will be the decision of the centres whether to receive international patients. We are working on bringing CTL019 to other countries around the world.” She added that the company would file for approvals in the EU later this year.
"By late November 2016, 11 of the 52 patients in the study who went into remission relapsed, 29 were still in remission and 11 others had further treatments, like bone marrow transplants. One patient was not available for assessment. Three who had relapses died, and one who did not relapse died from a new treatment given during remission. The median duration of remission is not known because it has not been reached: Some patients were still well when last checked, the report said.
"Researchers are still debating which patients can safely forgo further treatment, and which might need a bone marrow treatment to give the best chance of a cure.
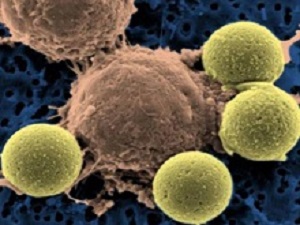
'The treatment requires removing millions of a patient’s T-cells — a type of white blood cell often called soldiers of the immune system — and genetically engineering them to kill cancer cells. The technique employs a disabled form of HIV, the virus that causes Aids, to carry new genetic material into the T-cells to reprogramme them. The process turbocharges the T-cells to attack B-cells, a normal part of the immune system that turn malignant in leukaemia. The T-cells home in on a protein called CD-19 that is found on the surface of most B-cells.
"The altered T-cells are then dripped back into the patient’s veins, where they multiply and start fighting the cancer," the article explains.
Dr Carl H June, a leader of the University of Pennsylvania team that developed the treatment, calls the turbocharged cells “serial killers”. A single one can destroy up to 100,000 cancer cells.
Because the treatment destroys not only leukemic B-cells but also healthy ones, which help fight germs, patients need treatment to protect them from infection. So every few months they receive infusions of immune globulins.
"In studies, the process of re-engineering T-cells for treatment sometimes took four months, and some patients were so sick that they died before their cells came back. At the meeting, Novartis said the turnaround time was now down to 22 days. The company also described bar-coding and other procedures used to keep from mixing up samples once the treatment is conducted on a bigger scale," the article reports.
Michael Werner, a lawyer and expert on gene and cell technologies and regulation, said that results so far proved that T-cell treatment works. “The fact that it can be done means more people will go into the field and more companies will start developing these products.” According to the report, he said: “I think we’re in for really exciting times.”
The treatment is part of one of the most important developments in cancer research in decades. And, says an NPR report, while it has generated much hope, there are some concerns about its safety over the long term – and its cost.
Even so, the report says, several of the committee members were unusually enthusiastic in explaining their 10-0 vote recommending approval. "This is the most exciting thing I've seen in my lifetime," said Dr Timothy Cripe, an oncologist at the Nationwide Children's Hospital in Columbus, Ohio. "This is a major advance and is ushering in a new era in treating children," agreed Dr Malcolm Smith, associate branch chief for paediatric oncology at the National Cancer Institute.
The report says for years, scientists have tried to use drugs that stimulate the immune system to fight cancer, and have had only modest success. In recent years, however, scientists developed a new generation of "immunotherapy" drugs that have produced impressive results for a wide range of cancers by unleashing the body's natural defence system.
The new treatment is known as CAR-T cell immunotherapy. It works by removing key immune system cells known as T cells from the patient so scientists can genetically modify them to seek out and attack only cancer cells. That's why some scientists refer to this as a "living drug." Doctors then infuse millions of the genetically modified T cells back into the patient's body so they can try to obliterate the cancer cells and hopefully leave healthy tissue unscathed.
"It's truly a paradigm shift," said Dr David Lebwohl, who heads the CAR-T Franchise Global Programme at Novartis. "It represents a new hope for patients."
"There is a major unmet medical need for treatment options" for B-cell acute lymphoblastic leukaemia patients, Dr Stephen Hunger, who helped study at the Children's Hospital of Philadelphia, told the committee.
Still, while those results are encouraging, the report says the approach has raised concerns. The treatment can produce a life-threatening adverse reaction known as a "cytokine release syndrome," in which the immune system attacks vital organs. In the past, a handful of patients who were getting similar treatments developed by other companies died from serious brain swelling. But although those sorts of complications did occur in some patients receiving CTL019, the patients recovered and there were no fatalities, the company says.
There are also concerns about possible long-term complications. Scientists use a virus to make the genetic changes in the T cells, raising fears about possible long-term side effects.
The report says because of the safety concerns, the company plans to continue to follow the medical progress of patients receiving the treatment. The company is also planning to initially make the treatment available only at about 30 to 35 hospitals that have had the necessary training and expertise to produce and administer the complex treatment.
Another big concern is the cost. While Novartis will not estimate the price it will ultimately put on the treatment, some industry analysts project it will cost $500,000 per infusion. Nevertheless, during a public comment period several family members of children who benefited from the treatment in its experimental phase have made emotional appeals to the committee to recommend approval.
"I'm happy most of all for the patients who will benefit from this therapy," said Dr Carl June of the University of Pennsylvania Perelman School of Medicine, who developed the therapy.
[link url="https://www.nytimes.com/2017/07/12/health/fda-novartis-leukemia-gene-medicine.html?emc=edit_th_20170713&nl=todaysheadlines&nlid=42505380"]The New York Times report[/link]
[link url="http://www.npr.org/sections/health-shots/2017/07/12/536812206/living-drug-that-fights-cancer-by-harnessing-the-immune-system-clears-key-hurdle?utm_source=nextdraft&utm_medium=email"]NPR report[/link]
