Big Pharma's hefty price tags for some medicines have again come under scrutiny, with a drug developed by Médecins Sans Frontières (MSF) costing a fraction of the billions claimed by drug-makers.
MedicalBrief writes that this comes as another drug, billed as the world's most expensive at the time of its launch, has failed to gain momentum, raising issues about its affordability for those who need it and whether pharmaceutical companies are justified in charging the prices they do.
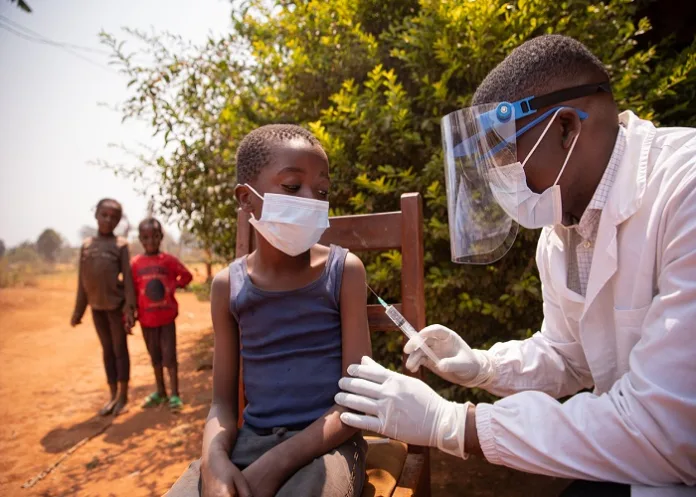
MSF is challenging pharmaceutical companies to be transparent about the cost of drug development after its own bill for a major trial of TB treatment cost a fraction of the billions claimed by drug-makers.
MSF’s bill for landmark trials of the four-drug combination treatment for drug-resistant TB came to €34m, reports The Guardian.
Current estimates for research and development of new medicines range from €40m to €3.9bn. The extortionate cost of trials is used to justify high prices of new medicines, but companies never publish either the topline or a breakdown of their spending.
MSF says this opacity should end.
It has produced a toolkit for drug triallists that categorises each item of expenditure and allows the costs to be collated throughout the process, which can last for years.
MSF’s trial, called TB Practecal, has transformed prospects for people with drug-resistant forms of TB, which have high mortality rates and in some countries have been untreatable because of the high price of the few drugs that still work.
Dr Bern-Thomas Nyang’wa, MSF’s medical director and the chief investigator of the trial, said: “We hope our disclosure of clinical trial costs … will serve as a clarion call for other public and non-profit actors to publicly share their clinical trial costs to ensure broader transparency in medical R&D costs.”
He said the organisation encouraged clinical trial sponsors and implementors to try the MSF’s Transparency Core toolkit, and develop it as a guide to facilitate the publication of cost data.
“While transparency in R&D expenditure remains largely elusive, transparency … is a transformative step towards exposing what medical innovation actually costs and building a future where access to medicines and medical tools is not hindered by high prices.”
The two antimicrobial drugs that have been the staple treatment for TB for decades, isoniazid and rifampicin, no longer work as well as they did. The outlook for patients with drug-resistant TB has been bleak in middle and low-income countries. Even if alternative drugs were available, they must be taken regularly for an entire year.
Bedaquiline, a new drug with a different mechanism against drug-resistant TB, was developed by Johnson & Johnson and in 2012, became the first TB drug to be approved by the FDA in the US in 40 years.
But the cost was prohibitive for many of the worst-affected countries.
Campaigners undertook a lengthy battle to get the price reduced. The cost of R&D was a key factor. Eventually, academics revealed that the drug was developed thanks to public funding, which was five times more than private investment.
MSP trial
MSF trialled the use of a combination of four oral drugs, including bedaquiline, against drug-resistant TB. Its success led to the World Health Organisation (WHO) recommending six months’ treatment with the combination for rifampicin-resistant TB. It is now used in 40 countries.
Roz Scourse, a policy adviser with MSF’s Access Campaign, said: “The global movement that pushed for a significant price reduction of bedaquiline demonstrated that transparency of R&D costs can lead to increased access to medical tools and help save more lives.
“The unsubstantiated yet dominant narrative that high prices are needed to recoup high R&D costs can no longer remain an evidence-free zone – this information is a critical piece of the policy puzzle that can inform the price of medical products, and who gets access.”
MSF’s paper, presented last week at a WHO conference on medicines pricing, showed it was possible to collect good data on spending in trials, Scourse said. She urged public disclosure, so that governments and communities can have the evidence they need to discuss pricing and work towards access for all those who need the medicines.
Although MSF’s trials took place in middle-income countries, the costs were not low, said Scourse, because they had to invest substantial sums to upgrade infrastructure – like TB clinics – to be able to conduct high-quality research.
The pharmaceutical industry trade body, the International Federation of Pharmaceutical Manufacturers and Traders (IFPMA), said most estimates for the cost of an approved drug ranged from $2.2bn-$3.2bn, and pointed to a Deloitte analysis from 2022 which put the average at $2.3bn.
“The pharmaceutical industry invests around $200bn every year on research and development,” said James Anderson, IFPMA’s executive director of global health.
“Over the past 10 years alone, companies have developed more than 470 medicines to treat diseases such as cancer, cardiovascular diseases and diabetes, as well as vaccines to protect against significant infections like malaria, RSV and Covid-19, among others.
“Medicines should be affordable to healthcare systems, available to patients everywhere.”
One medicine which has come under scrutiny is Zolgensma, a one-off treatment for infants born with a rare and often fatal genetic disease, which came to the market five years ago at an eye-watering $2.1m. But its Swiss manufacturer Novartis is now under growing pressure as annual sales decline and new markets prove tough to crack.
Launched with great fanfare in 2019, reports SwissInfo, it was the first gene therapy to treat spinal muscular atrophy (SMA) – a rare neuromuscular disorder and the leading genetic cause of infant mortality worldwide.
The one-time infusion was originally touted as a potential cure for SMA and one of the Swiss pharmaceutical giant’s six major growth drivers with multibillion-dollar sales potential.
In 2021, sales jumped 46% to $1.35bn after the European Medicines Agency gave conditional authorisation for the treatment in 2020.
But Zolgensma, the brand name for the drug onasemnogene abeparvovec, has failed to maintain this momentum.
One key reason is that “established markets, like the US, are treating mainly incident versus prevalent patients now”, a Novartis spokesperson said.
In other words, the company has already reached existing eligible SMA patients and in future can only rely on newly diagnosed patients to support the drug’s sales growth in these markets.
Other markets outside the relatively wealthy countries of the US and Europe have been difficult to penetrate as governments with tight healthcare budgets question whether the drug is effective enough to justify its price tag.
How can a drug cost $2.1m?
The US Food and Drug Administration (FDA) was the first to give the Zolgensma the green light in 2019 when it was priced by Novartis at a staggering $2.1m, making it at the time the most expensive one-time treatment ever.
Although Zolgensma has been approved in 51 countries as of January 2024 and has treated more than 3 700 patients, only 35 countries offer commercial reimbursement, leaving many SMA sufferers unable to afford a potentially life-saving drug or having to resort to crowdfunding to raise the cash.
Justifying the price
SMA affects around one in 10 000 infants globally, which is around 300 babies a year in the US. It is caused by a gene mutation called SMN1, which prevents cells from producing enough of the protein SMN that sends vital signals to muscles. The shortfall impairs motor neurons, causing muscles to weaken.
Without treatment, babies with the most severe form of SMA might never be able to lift their heads or legs, and struggle to swallow and breathe. Most die by the age of two, often due to respiratory issues. SMA symptoms can also develop in adults but are less severe.
Drugs to treat rare diseases like SMA can take years and billions of dollars to develop.
Novartis justified Zolgensma’s price tag by arguing that it was a one-time infusion targeting the genetic root cause of the disease. By replacing a faulty gene with a functioning one, the drug would not only save lives but spare families and health systems a lifetime of costly treatment.
Before the gene therapy hit the market, the only other available treatment for SMA was nusinersen, launched as Spinraza by US company Biogen in 2016 and which costs $300 000 to $500 000 per year over a patient’s lifetime.
If taken for a decade, the drug, which is given as a spinal injection three times a year, would far exceed Zolgensma’s $2.1m list price.
Novartis hasn’t explained how it calculated Zolgensma’s price and independent evaluations offer wildly different estimates of how much it should cost – from $710 000 to more than $2.1m – adding to the controversy over the company’s rationale for charging so much.
The fact that so few patients had received the drug when it was priced also raised questions about its true value. Like many treatments for life-threatening rare diseases, Zolgensma was given the green light in the US, Europe and Japan on an accelerated approval timeline, which gives patients faster access to a potentially life-saving drug.
The FDA based its decision on a completed early phase and an ongoing late-stage trial involving fewer than 40 patients in total. Most of the infants who took Zolgensma survived, could breathe on their own, and reached milestones like being able to sit up without support two years after taking the drug.
But progress varied significantly and some children experienced serious side effects, including liver problems. The short timeframe also meant there was no data on how long the benefits of the drug would last.
There were also few participants outside the US in trials.
The US and some wealthy countries limited Zolgensma approval to the youngest patients (under two-years-old) with the most severe form of SMA and where there was the strongest evidence of its safety and efficacy.
The European Medicines Agency authorised the drug for children who weighed up to 21kg, which could include toddlers as old as five, but many member states would only reimburse treatment for those under six-months-old.
The high-price hurdle
With limited growth potential in established markets, expanding Zolgensma’s geographic reach has become crucial for Novartis. But given competing health demands and limited budgets, many middle-income countries want more evidence that the drug justifies the asking price.
“With rare diseases, you have the challenge that you need access rapidly but at the same time you have drugs with extremely high prices and many uncertainties. Sometimes you don’t really know the real clinical benefits,” said Vera Pepe, a public health researcher and co-author of a paper on access to Zolgensma in Brazil.
“Companies can no longer just come to the market with a two-million-dollar drug and expect governments to pay for it,” said Girisha Fernando, the CEO of Lyfegen, a global provider of drug pricing and rebate management solutions. “They need to offer more than that.”
Novartis has said it wants to find ways to make Zolgensma accessible in middle-income countries, and is offering discounts, instalment payment plans and risk-sharing agreements to insurance providers and national health authorities, where payment is dependent on pre-agreed outcomes.
Last year, the company signed such a deal with Argentina’s national health payer to provide Zolgensma at $1.3m.
Payment will only be made if “results observed in the patients match what is expected according to available scientific evidence”.
For the families of children with SMA, the wait for access is agonising. The longer children have to forego treatment, the more their condition deteriorates and cannot be reversed with Zolgensma.
Data dilemma
Novartis has been helped to some extent by the results of more clinical trials and so-called real-world data from healthcare providers globally that show positive outcomes for many patients receiving the therapy.
In March 2023, Novartis’ own studies showed that patients who took Zolgensma as infants maintained major motor milestones, such as sitting up and being able to breathe without assistance seven years after taking the drug.
Older children with less severe forms of the disease – who received a newer version of the drug at a higher dose – also improved.
However, some patients stopped making progress and 24 of 81 children in the studies were subsequently treated with other SMA drugs.
Other studies, including one in Switzerland involving nine patients, underscore the huge range of possible outcomes.
The growing body of trial and treatment results shows that the initial hype over Zolgensma as a one-time cure for SMA sufferers has not been borne out in reality.
Nicole Gusset, who heads the patient advocacy groups SMA Europe and SMA Switzerland and has a daughter with SMA, told SWI:“Cure is a big word and must be used sparingly. A stabilisation of the progression of a disease or the absence of visible symptoms are enormous successes but do not automatically mean a disease is cured.”
Novartis also faces more pressure as other drugs that treat SMA enter the market. In 2020, the US FDA approved ridisplam, sold as Evrysdi, from Swiss rival Roche, for older children and adults with SMA. The approval was recently expanded to cover infants younger than two months, making it a more direct challenger to Zolgensma.
Evrysdi comes in pill and syrup form, making it easier to administer than a gene therapy, and costs $100 000-$350 000 annually, depending on the patient’s weight. Evrysdi has been approved in more than 100 countries and used to treat more than 11 000 SMA sufferers globally.
The right price
Other gene therapy developers, who are pouring billions into new drugs, are watching closely how the Swiss pharma giant navigates these headwinds. There are more than 1 500 clinical trials for cell and gene therapies registered with US authorities and last year the FDA approved five, including two for sickle cell disease that had list prices higher than Zolgensma – at $2.2m and $3.1m.
In March, Orchard Therapeutics set a new record with a price tag of $4.25m in the US for Lenmeldy (also called Libmeldy), a gene therapy for metachromatic leukodystrophy, an ultra-rare genetic condition that attacks the central nervous system of children. The disease affects roughly 40 newborn babies a year in the US.
Can drugs be priced fairly?
Gene therapies are already disappearing because of price disputes. At least seven out of 25 approved cell and gene therapies have been withdrawn from the market in Europe. The most prominent was Zynteglo, a treatment for the blood disorder beta-thalassemia. Its maker, US-based Bluebird Bio, closed its EU operations after multiple disputes over price.
“A big worry is that drugs are being removed from the market because they are too expensive or aren’t selling,” said Kelly Ormond, a genetic counsellor and researcher at the Health Ethics and Policy Lab at the federal technology institute ETH Zurich. “If we want to be able to take advantage of the promise of precision medicine like gene therapies, we have to sort out how we pay for them.”
Swiss Info – Whatever happened to the world’s most expensive drug?
See more from MedicalBrief archives:
Non-profits take over from Big Pharma in new drugs trials
Experts claim that Big Pharma hides true drug development costs
Poor people also have a right to medicine
SA in multi-million dollar trial for new TB vaccine
FDA approves ‘world’s costliest drug’ for children with MLD