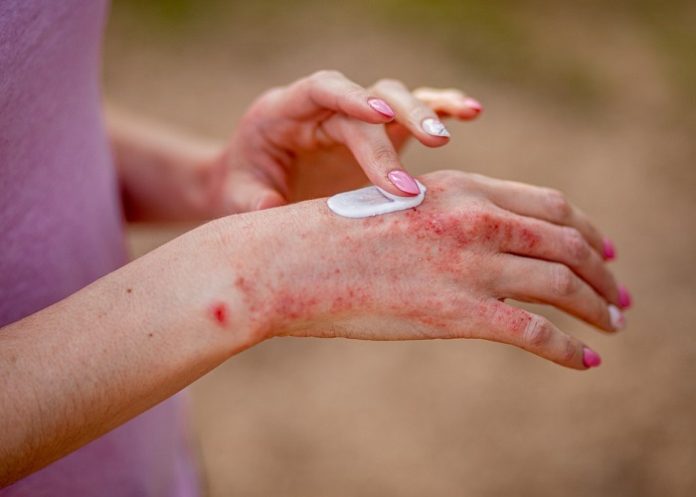
The FDA has approved a new treatment as the first and only skin cream for a specific type of hand eczema that affects about one in every 10 people globally, reports WebMD.
The cream, sold under the name Anzupgo, contains a new drug called delgocitinib and is for adults with long-term or chronic hand eczema (CHE), especially if steroid creams don’t help or aren’t a good option.
CHE affects hands and wrists, causing the skin to become dry, itchy, sore, blistered, thick, or swollen. It can last for at least three months and flares up at least twice yearly – developing when the skin’s protective barrier is damaged, and leading to inflammation and changes in the naturally existing skin bacteria.
The condition can seriously affect daily life and mental health, as around 70% of people with severe CHE find it hard to do everyday tasks.
Currently available treatments often provide only temporary relief, may cause side effects with long-term use, and may need to be injected, highlighting the need for effective and easy-to-use options.
Anzupgo was tested in two studies with about 960 people who had moderate to severe CHE. They used either Anzupgo or a placebo cream twice a day for 16 weeks. More people who used Anzupgo had clear or almost clear skin (20% and 29%), compared with those who used the fake cream (10% and 7%).
In a longer follow-up study, about 30% of patients using Anzupgo continued to see improvement after 36 weeks. Overall, the treatment was well-tolerated. Less than 1% of them reported side effects like mild skin pain, tingling, itching, redness, or skin infections. A few also had low white blood cell counts.
Anzupgo, made by Leo Pharma, is a steroid-free cream that works by blocking key signals from specific proteins in the immune system known as JAK, which cause inflammation in the skin when over-activated. This helps reduce flare-ups and relieve symptoms.
The FDA advises against using Anzupgo alongside other medications that work in a similar way or suppress the immune system, as this could raise the risk of side effects.
People using Anzupgo should also avoid live vaccines right before, during, and shortly after treatment, and breastfeeding women are told not to touch their nipple area after applying the cream to their hands or wrists.
Doctors should be informed of any ongoing or frequent infections, as well as any other health conditions that might increase the risk of infection during treatment.
WebMD article – FDA OKs New Steroid-Free Skin Cream for Hand Eczema (Open access)
See more from MedicalBrief archives:
Drug trial shows relief for infants, children from eczema’s itch – US randomised study
Air pollution linked to higher eczema risk – Yale study
Development of microbe for bacteriotherapy of eczema — First-in-human trial