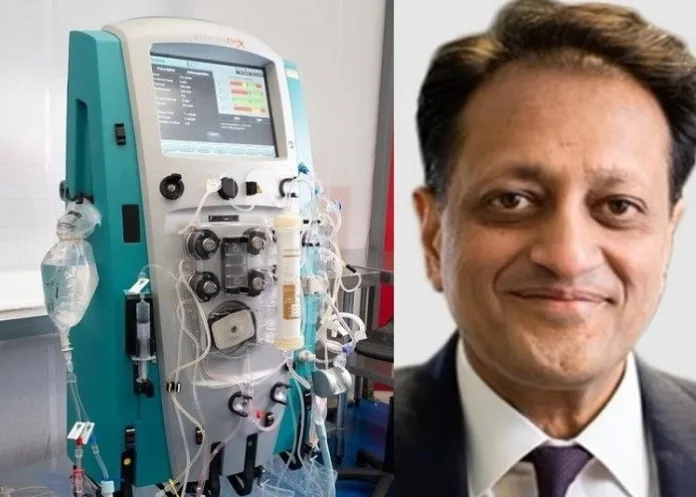
A dialysis machine that can cure liver failure has been developed by British scientists in a major boost for millions of patients, with early trials finding it stopped twice as many of them from going into organ failure as did existing treatments, eliminating the need for transplants.
Developed by University College London researchers, the device filters toxins from the blood of patients with liver failure, allowing the liver time to regenerate itself in as little as a month.
Experts hope it could be rolled out in NHS hospitals within three years by modifying existing kidney dialysis equipment, if further trials are successful, reports Daily Mail.
Professor Rajiv Jalan, of the UCL Institute for Liver and Digestive Health who invented Dialive, said it was the culmination of “50 years of failure”.
“As academics, it can be difficult to define a disease and then translate this knowledge into a clinical solution that makes a real difference to people’s lives. So the results of the Dialive trial are an emotional moment. It is built on the learnings from many failures in the system over the past 30, 40, 50 years.”
Working with the Royal Free Hospital, 32 patients were given dialysis or standard care for up to five days.
The treatment works using two novel filters to clean the blood in a similar way to how kidney dialysis is currently performed. The procedure, which lasts from eight to 12 hours, meant that around twice as many patients came out of liver failure as those on standard drugs treatment.
The early results, published in the Journal of Hepatology, suggest patients will only need between five and 10 courses of dialysis, turning the tide on the disease in as little as a month.
Despite receiving just three days’ treatment, patients whose liver failure resolved remained in remission for 28 days afterwards.
“We started to understand what accumulates in the body when the liver is failing – nasty, toxic substances which the body is not able to clear that lead to further liver failure and failure of regeneration,” said Jalan.
“The liver has an incredible potential to regenerate. If we can keep the patient alive for long enough and clear the environment for liver regeneration to happen, then we should be able to bridge many of these patients for recovery, because normal healing processes will take over.
“For a substantial number of patients, it will prevent the need for liver transplantation.”
Scientists hope to begin further trials within months.
Study details
Randomised, controlled clinical trial of the DIALIVE liver dialysis device versus standard of care in patients with acuteon-chronic liver failure
Banwari Agarwal, Rafael Bañares Cañizares, Rajiv Jalan, et al.
Published in the Journal of Hepatology on 1 June 2023
Background & Aims
Acute-on-chronic liver failure (ACLF) is characterised by severe systemic inflammation, multi-organ failure and high mortality rates. Its treatment is an urgent unmet need. DIALIVE is a novel liver dialysis device that aims to exchange dysfunctional albumin and remove damage- and pathogen-associated molecular patterns. This first-in-man randomised controlled trial was performed with the primary aim of assessing the safety of DIALIVE in patients with ACLF, with secondary aims of evaluating its clinical effects, device performance and effect on pathophysiologically relevant biomarkers.
Methods
Thirty-two patients with alcohol-related ACLF were included. Patients were treated with DIALIVE for up to 5 days and end points were assessed at Day 10. Safety was assessed in all patients (n = 32). The secondary aims were assessed in a pre-specified subgroup that had at least three treatment sessions with DIALIVE (n = 30).
Results
There were no significant differences in 28-day mortality or occurrence of serious adverse events between the groups. Significant reduction in the severity of endotoxemia and improvement in albumin function was observed in the DIALIVE group, which translated into a significant reduction in the CLIF-C (Chronic Liver Failure consortium) organ failure (p = 0.018) and CLIF-C ACLF scores (p = 0.042) at Day 10. Time to resolution of ACLF was significantly faster in DIALIVE group (p = 0.036). Biomarkers of systemic inflammation such as IL-8 (p = 0.006), cell death [cytokeratin-18: M30 (p = 0.005) and M65 (p = 0.029)], endothelial function [asymmetric dimethylarginine (p = 0.002)] and, ligands for Toll-like receptor 4 (p = 0.030) and inflammasome (p = 0.002) improved significantly in the DIALIVE group.
Conclusions
These data indicate that DIALIVE appears to be safe and impacts positively on prognostic scores and pathophysiologically relevant biomarkers in patients with ACLF. Larger, adequately powered studies are warranted to further confirm its safety and efficacy.
See more from MedicalBrief archives:
Lack of dialysis Tx in sub-Saharan Africa raises ethical questions
NHS nurse walkout could delay chemo, dialysis and urgent procedures
Millions worldwide not getting dialysis