 Machine learning has detected one of the commonest causes of dementia and stroke, in the most widely used form of brain scan (CT), more accurately than current methods. New software, created by scientists at Imperial College London and the University of Edinburgh, has been able to identify and measure the severity of small vessel disease, one of the commonest causes of stroke and dementia. The study took place at Charing Cross Hospital, part of Imperial College Healthcare NHS Trust.
Machine learning has detected one of the commonest causes of dementia and stroke, in the most widely used form of brain scan (CT), more accurately than current methods. New software, created by scientists at Imperial College London and the University of Edinburgh, has been able to identify and measure the severity of small vessel disease, one of the commonest causes of stroke and dementia. The study took place at Charing Cross Hospital, part of Imperial College Healthcare NHS Trust.
Researchers say that this technology can help clinicians to administer the best treatment to patients more quickly in emergency settings – and predict a person's likelihood of developing dementia. The development may also pave the way for more personalised medicine.
Dr Paul Bentley, lead author and clinical lecturer at Imperial College London, said: "This is the first time that machine learning methods have been able to accurately measure a marker of small vessel disease in patients presenting with stroke or memory impairment who undergo CT scanning. Our technique is consistent and achieves high accuracy relative to an MRI scan – the current gold standard technique for diagnosis. This could lead to better treatments and care for patients in everyday practice."
Professor Joanna Wardlaw, head of neuroimaging sciences at the University of Edinburgh, added: "This is a first step in making a scan reading tool that could be useful in mining large routine scan datasets and, after more testing, might aid patient assessment at hospital admission with stroke."
Small vessel disease (SVD) is a very common neurological disease in older people that reduces blood flow to the deep white matter connections of the brain, damaging and eventually killing the brain cells. It causes stroke and dementia as well as mood disturbance. SVD increases with age but is accelerated by hypertension and diabetes.
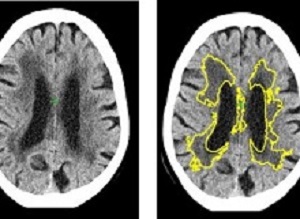
At the moment, doctors diagnose SVD by looking for changes to white matter in the brain during MRI or CT scans. However, this relies on a doctor gauging from the scan how far the disease has spread. In CT scans it is often difficult to decide where the edges of the SVD are, making it difficult to estimate the severity of the disease, explains Bentley.
Although MRI can detect and measure SVD more sensitively it is not the most common method used due to scanner availability, and suitability for emergency or older patients. Bentley added: "Current methods to diagnose the disease through CT or MRI scans can be effective, but it can be difficult for doctors to diagnose the severity of the disease by the human eye. The importance of our new method is that it allows for precise and automated measurement of the disease. This also has applications for widespread diagnosis and monitoring of dementia, as well as for emergency decision-making in stroke."
Bentley explains that this software could help influence doctors decision-making in emergency neurological conditions and lead to more personalised medicine. For example, in stroke, treatments such as 'clot busting medications' can be quickly administered to unblock an artery. However, these treatments can be hazardous by causing bleeding, which becomes more likely as the amount of SVD increases. The software could be applied in future to estimate the likely risk of haemorrhage in patients and doctors can decide on a personal basis, along with other factors, whether to treat or not with clot busters.
He also suggests that the software can help quantify the likelihood of patients developing dementia or immobility, due to slowly progressive SVD. This would alert doctors to potentially reversible causes such as high blood pressure or diabetes.
The study used historical data of 1082 CT scans of stroke patients across 70 hospitals in the UK between 2000-2014, including cases from the Third International Stroke Trial. The software identified and measured a marker of SVD, and then gave a score indicating how severe the disease was ranging from mild to severe. The researchers then compared the results to a panel of expert doctors who estimated SVD severity from the same scans. The level of agreement of the software with the experts was as good as agreements between one expert with another.
Additionally, in 60 cases they obtained MRI and CT in the same subjects and used the MRI to estimate the exact amount of SVD. This showed that the software is 85% accurate at predicting how severe SVD is.
The team are now using similar methods to measure the amount of brain shrinkage and other types of conditions commonly diagnosed on brain CT.
The study was funded by a National Institute for Health Research i4i Invention for Innovation award, and a National Institute for Health Research Imperial Biomedical Research Centre grant (NIHR BRC).
Abstract
Purpose: To validate a random forest method for segmenting cerebral white matter lesions (WMLs) on computed tomographic (CT) images in a multicenter cohort of patients with acute ischemic stroke, by comparison with fluid-attenuated recovery (FLAIR) magnetic resonance (MR) images and expert consensus.
Materials and Methods: A retrospective sample of 1082 acute ischemic stroke cases was obtained that was composed of unselected patients who were treated with thrombolysis or who were undergoing contemporaneous MR imaging and CT, and a subset of International Stroke Thrombolysis–3 trial participants. Automated delineations of WML on images were validated relative to experts’ manual tracings on CT images, and co-registered FLAIR MR imaging, and ratings were performed by using two conventional ordinal scales. Analyses included correlations between CT and MR imaging volumes, and agreements between automated and expert ratings.
Results: Automated WML volumes correlated strongly with expert-delineated WML volumes at MR imaging and CT (r2 = 0.85 and 0.71 respectively; P < .001). Spatial-similarity of automated maps, relative to WML MR imaging, was not significantly different to that of expert WML tracings on CT images. Individual expert WML volumes at CT correlated well with each other (r2 = 0.85), but varied widely (range, 91% of mean estimate; median estimate, 11 mL; range of estimated ranges, 0.2–68 mL). Agreements (κ) between automated ratings and consensus ratings were 0.60 (Wahlund system) and 0.64 (van Swieten system) compared with agreements between individual pairs of experts of 0.51 and 0.67, respectively, for the two rating systems (P < .01 for Wahlund system comparison of agreements). Accuracy was unaffected by established infarction, acute ischemic changes, or atrophy (P > .05). Automated preprocessing failure rate was 4%; rating errors occurred in a further 4%. Total automated processing time averaged 109 seconds (range, 79–140 seconds).
Conclusion: An automated method for quantifying CT cerebral white matter lesions achieves a similar accuracy to experts in unselected and multicenter cohorts.
Authors
Liang Chen, Anoma Lalani Carlton Jones, Grant Mair, Rajiv Patel, Anastasia Gontsarova, Jeban Ganesalingam, Nikhil Math, Angela Dawson, Basaam Aweid, David Cohen, Amrish Mehta, Joanna Wardlaw, Daniel Rueckert, Paul Bentley
[link url="http://www.imperial.ac.uk/news/186108/artificial-intelligence-improves-stroke-dementia-diagnosis/"]Imperial College London material[/link]
[link url="https://pubs.rsna.org/doi/10.1148/radiol.2018171567"]Radiology abstract[/link]
