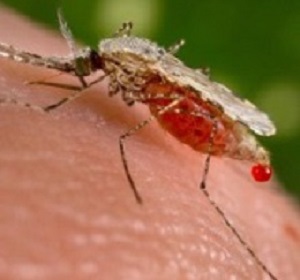
 Seasonal malaria chemo-prevention is saving thousands of children in West Africa, according to studies presented this week at the American Society of Tropical Medicine and Hygiene (ASTMH) meeting in Baltimore, Maryland.
Seasonal malaria chemo-prevention is saving thousands of children in West Africa, according to studies presented this week at the American Society of Tropical Medicine and Hygiene (ASTMH) meeting in Baltimore, Maryland.
In a sea of high-tech malaria fixes – everything from drug-delivery by drone to gene-edited mosquitoes – this old-fashioned approach involves giving children a dose of antimalarial drugs once each month in the rainy season to prevent the disease in hard-hit regions. Researchers have previously demonstrated this strategy in large clinical trials but they had feared that their positive results wouldn’t be replicated in the messy, real world, because chemo-prevention requires thousands of local health workers to deliver drugs to children in villages far from hospitals, pharmacies and paved roads.
“People were doubtful this intervention would work, because it’s so demanding,” says Brian Greenwood, an infectious disease specialist at the London School of Hygiene and Tropical Medicine who helped to conduct trials that showed reductions in malaria prevalence up to 84%. As a result of those studies, more than 6.4m children in nine countries in sub-Saharan Africa (Burkina Faso, Cameroon, Chad, Gambia, Guinea, Mali, Niger, Nigeria, Senegal) received the drugs in 2016.
It seems to be working, according to data presented at the ASTMH meeting. “They are seeing the same level of efficacy against malaria that we saw in clinical trials and reducing hospital admissions,” says Greenwood. “I am very happy.” But researchers are also finding signs that this approach may not work for long.
Malaria researchers deployed chemo-prevention in the 1950s, but it fell out of favour when the widespread use of malaria drugs led to drug resistance. Yet by 2000, more than 830,000 people were dying of the disease each year – mainly children in Africa – and there were no blockbuster vaccines on the horizon. So malariologists revisited the approach. Between 2002 and 2012, clinical trials conducted in West Africa suggested that combinations of older malaria drugs had the power to prevent 8.8m cases and 80,000 deaths every year if implemented solely during the rainy season, when the disease spikes.
In 2012, the World Health Organisation recommended the strategy with three old drugs – sulphadoxine, pyrimethamine and amodiaquine – so that the only sure-fire cure for malaria, artemisinin, would remain effective. Alassane Dicko, a malariologist at the University of Bamako in Mali, says that he did not take the intervention for granted when it launched in Mali in 2013, because he knew that funds were limited and drug resistance inevitable. “Research is essential,” he says. His lab began assessing chemo-prevention’s efficacy, cost and effects on drug resistance.
In August, Dicko and his colleagues reported that malaria prevalence was reduced by 65% in children under age 5 who were treated with chemo-prevention in the Malian district of Kita, compared to a similar number of children in a neighbouring district that lacked the funds to roll out the intervention.
On the basis of results such as these, malaria researchers at the meeting estimate that chemo-prevention has averted roughly 6m cases and 40,000 deaths in 2015 and 2016 in the countries where it is practised. “This intervention has been extremely well documented over three or four years,” says Erin Eckert, an epidemiologist at the US Agency for International Development’s President’s Malaria Initiative, based in Washington DC. As a result, the agency plans to help fund chemo-prevention in eight countries next year.
Also at the ASTMH meeting, Dicko reported a 80-person trial showing that adding another old malaria drug, primaquine, to the regimen combo blocks the transfer of the malaria parasite, Plasmodium falciparum, from humans into mosquitoes. This would further reduce the amount of the parasite in circulation. Dicko aims to hit the disease hard and fast – with multiple drugs, as soon as possible – because he and his colleagues are already detecting genetic signs of drug resistance in parasites.
New chemo-prevention drugs in the pipeline might not be ready before existing drugs fail because of resistance, Greenwood says. This year, he helped to launch a trial combining chemo-prevention and a less effective malaria vaccine in Burkina Faso and Mali. The vaccine was previously shown to reduce the number of malaria cases by less than 36% in children, but Greenwood hopes the combined tools, together with bed nets, can suppress malaria enough to stop it from bouncing back once today’s drugs fail. By that time, he says, genetically engineered mosquitoes might be ready to fly.
Abstract
Ivermectin mass drug administration (MDA) to humans has been proposed as a novel transmission control tool measure to aid global malaria elimination efforts. Field trials in West Africa have indicated that ivermectin MDAs can suppress malaria transmission as measured by mosquito and human parameters. However, there is discordance between in vitro mosquito survivorship assays, pharmacokinetic predictions of ivermectin and results from clinical trials. Recent clinical trial evidence indicates that ivermectin treatment of humans has much greater mosquito-lethal impact than initially predicted. This suggests that there may be ivermectin metabolites with mosquito-lethal properties which extend pharmacodynamic effects beyond what the parent compound predicts. It is critical to evaluate and quantify the pharmacokinetic and pharmacodynamic (PK-PD) relationship of human treatment and mosquito killing duration. Two clinical trials have evaluated the pharmacokinetic interaction and mosquito-lethal efficacy of ivermectin and dihydroartemisinin-piperaquine (DHA-PQP) on Anopheles survival in Thailand and Kenya. These ivermectin PK-PD results will provide the basis for development of novel drug co-formulations and long-lasting drugs to enhance and extend the mosquito-lethal and therapeutic effects of ivermectin. Current ivermectin formulations as a single dose during MDA can interrupt malaria transmission but this effect could be greater with novel strategies. The dose used for onchocerciasis and lymphatic filariasis MDAs is based on weight. Weight-based dosing of ivermectin hampers the possibility to co-formulate with other fixed-dose drugs. Ongoing studies have evaluated novel single-dose tablets (18 or 36 mg) that would result in the population receiving a wide dosage range rather than a target weight-based dosage. Recently, an oral, ultra-long-acting capsule that can release ivermectin for days to weeks and potentially longer has been developed. Advances presented here include development of an animal model and planning of first human trials. Both novel ivermectin formulations reduce logistical issues during MDA. A mathematical model describing the impact of ivermectin on malaria transmission has been developed to translate the PK-PD data into estimates of potential public health impact. Using new data, efforts have focused on extending the pharmacodynamic component of the model to capture the observed discrepancy between ivermectin levels in the blood and mosquito killing efficacy. The model can inform where a higher dose or novel formulation of ivermectin may be particularly effective, based on the different levels of transmission intensity, seasonality and vector dynamics.
[link url="https://www.nature.com/news/resurrected-malaria-strategy-saves-thousands-of-lives-in-africa-1.22982"]Nature material[/link]
[link url="http://www.abstractsonline.com/pp8/#!/4395/session/126"]ASTMH Annual Meeting abstracts[/link]
