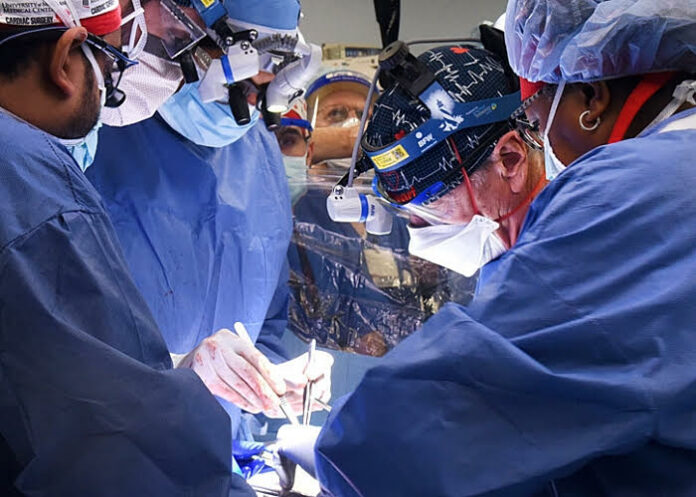
In a final effort to save a 57-year-old man’s life, doctors at the University of Maryland (UMSOM) have given him a genetically modified pig-heart transplant, writes MedicalBrief. The groundbreaking procedure, performed last Friday (7 January) will offer hope to hundreds of thousands of patients with failing organs.
Editor’s note: This story was updated on Friday 14 January.
The US Food and Drug Administration (FDA), which oversees xenotransplantation experiments (the transplant of animal organs), allowed the surgery under a “compassionate use” emergency authorisation. The historic surgery was conducted by the University of Maryland School of Medicine (UMSOM) faculty at the University of Maryland Medical Center (UMMC), together known as the University of Maryland Medicine.
The patient was doing well, said the medical team on Thursday (13 January), although it was too soon to know if the operation has been a success. He was breathing on his own while still connected to a heart-lung machine to help his new heart.
“It creates the pulse, it creates the pressure, it is his heart,” said Dr Bartley Griffith, director of the cardiac transplant programme at the medical centre who performed the heart transplant. “Itʼs working and it looks normal,” he told The New York Times. “We are thrilled, but we donʼt know what tomorrow will bring us. This has never been done before.”
The surgery took seven hours. Griffith said the patientʼs condition — heart failure and an irregular heartbeat — made him ineligible for a human heart transplant or a heart pump. Griffith had transplanted pig hearts into about 50 baboons over the past five years, before offering the option to David Bennett.
Bennett, a handyman, knew there was no guarantee the experiment would work but he was dying, ineligible for a human heart transplant and had no other option, his son said.
“It was either die or do this transplant. I want to live. I know itʼs a shot in the dark, but itʼs my last choice,” Bennett said a day before the surgery, according to a statement provided by UMSOM.
He had been admitted to the hospital more than six weeks earlier with life-threatening arrhythmia and was connected to a heart-lung bypass machine, called extracorporeal membrane oxygenation (ECMO), to remain alive. In addition to not qualifying to be on the transplant list, he was also deemed ineligible for an artificial heart pump due to his arrhythmia.
There is a massive shortage in US of human organs donated for transplant, driving scientists to try to figure out how to use animal organs instead. Last year, there were just more than 3,800 heart transplants in the the country, a record number, according to the United Network for Organ Sharing (Unos), which oversees the nationʼs transplant system.
About 110,000 Americans are currently waiting for an organ transplant, and more than 6,000 patients die each year before getting one, according to the federal government’s organdonor.gov.
This transplant marks a step in the decades-long quest to use animal organs for life-saving operations, reports available when a patient with a life-threatening condition has no other options, reports The Guardian. Doctors said the procedure showed that a heart from a genetically modified animal can function in the human body without immediate rejection.
“If this works, there will be an endless supply of these organs for patients who are suffering,” said Prof Muhammad Mohiuddin, scientific director of the universityʼs animal-to-human transplant programme. Considered one of the world's foremost experts on transplanting animal organs, known as xenotransplantation, Mohiuddin, professor of surgery at UMSOM, joined the faculty five years ago and established the Cardiac Xenotransplantation Program with Griffith.
Previous attempts at such transplants have failed, largely because patientsʼ bodies rapidly rejected the animal organ, notably, in 1984, with Stephanie Fae Beauclair (known as Baby Fae) at Loma Linda University in California. The infant, born with a fatal heart condition, received a baboon heart transplant and died within 21 days of the procedure due to the immune system’s rejection of the foreign heart. However, for many years, pig heart valves have been used successfully for replacing valves in humans.
Bennett had received one about a decade ago. The Maryland surgeons said the difference this time was that they had used a heart from a pig that had undergone gene-editing to remove a sugar in its cells thatʼs responsible for that hyper-fast organ rejection.
“I think you can characterise it as a watershed event,” Dr David Klassen, Unosʼ chief medical officer. Still, he cautioned that itʼs only a first tentative step into exploring whether this time around, xenotransplantation might finally work.
Several biotech companies are developing pig organs for human transplant; the one used for Bennett’s operation came from Revivicor, a subsidiary of United Therapeutics. Revivicor, a regenerative medicine company based in Blacksburg, provided the genetically-modified pig to the xenotransplantation laboratory at UMSOM. On the morning of the transplant surgery, the surgical team, led by Griffith and Mohiuddin, removed the pig's heart and placed it in the XVIVO Heart Box, a machine that keeps the heart preserved until surgery.
The physician-scientists also used a new drug along with conventional anti-rejection drugs, which are designed to suppress the immune system and prevent the body from rejecting the foreign organ. The new drug used is an experimental compound made by Kiniksa Pharmaceuticals.
Last September, researchers in New York performed an experiment in which they temporarily attached a pigʼs kidney to a deceased human body and watching it begin to work. The Maryland transplant takes their experiment to the next level, said Dr Robert Montgomery, who led that experiment at NYU Langone Health.
“This is a truly remarkable breakthrough,” he said. “As a heart transplant recipient, myself with a genetic heart disorder, I am thrilled by this news and the hope it gives to my family and other patients who will eventually be saved by this breakthrough.”
Organs from genetically modified pigs have been the focus of much of the research in xenotransplantation, in part because of physiologic similarities between pigs, human, and nonhuman primates. UMSOM received a $15.7m sponsored research grant to evaluate Revivicor genetically-modified pig UHearts™ in baboon studies.
Three genes, responsible for rapid antibody-mediated rejection of pig organs by humans, were “knocked out” in the donor pig. Six human genes responsible for immune acceptance of the pig heart were inserted into the genome. Last, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totalled 10 unique gene edits made in the donor pig, said Mohiuddin, who with Griffith, did much of the research leading up to the transplant.
Xenotransplantation, the process of grafting or transplanting organs or tissues from animals to humans, has a long history. Efforts to use the blood and skin of animals go back hundreds of years.
In the 1960s, chimpanzee kidneys were transplanted into some human patients, but the longest a recipient lived was nine months. Pigs offer advantages over primates for organ procurements because they are easier to raise and achieve adult human size in six months. Pig heart valves are routinely transplanted into humans, and some patients with diabetes have received porcine pancreas cells. Pig skin has also been used as a temporary graft for burn patients.
Dr Jay Fishman, associate director of the transplantation centre at Massachusetts General Hospital, said that using pig organs provides the ability to perform genetic manipulations, the time to carry out better screening for infectious diseases, and the possibility of a new organ when the patient needs it. “There are challenges for sure, but also opportunities,” he said.
It will be crucial to share the data gathered from this transplant before opening the option to more patients, said Karen Maschke, a research scholar at the Hastings Center, who is helping to develop ethics and policy recommendations for the first clinical trials under a grant from the National Institutes of Health.
“Rushing into animal-to-human transplants without this information would not be advisable,” she said.
Bennett's son said his father had realised the magnitude of what was done, and the importance of it. “My dad was on his deathbed,” said David Bennett Jr. “I mean, his prognosis early on was very, very bad. And the doctors have done everything in their power to keep him alive. They basically said he had less than six months and that this was very experimental. He could live or he could last a day or he could last a couple of days. Weʼre in the unknown at this point. It was very difficult for him to make this decision. But this was his best hope of getting out of the hospital and having somewhat of a normal quality of life.”
Bennett’s prognosis is uncertain and he is also being monitored for infections, including porcine retrovirus, a pig virus that may be transmitted to humans, although the risk is considered low.
The groundbreaking surgery has not been celebrated by everyone, however. According to a Washington Post report, in 1988, Bennett was convicted of stabbing one Edward Shumaker seven times and spent half a dozen years in prison.
The man’s sister, Leslie Shumaker Downey, said he was paralysed after the attack and used a wheelchair for the next 19 years. He had a stroke in 2005 and died a week before his 41st birthday in 2007.
“Ed suffered,” Downey told the Washington Post. “My family had to deal with the devastation and the trauma for years and years.”
Bennett was ordered by a court to pay Shumaker and his family $3.4m in damages, but Downey said her family never received a cent. Bennett “went on and lived a good life” after six years in prison, Downey added.“Now he gets a second chance with a new heart — but I wish, in my opinion, it had gone to a deserving recipient.”
Medical ethics experts, however, say that a patient’s criminal history should not factor into decisions about transplants, according to Business Insider.
Hospitals generally don’t take criminal history into account when choosing who to place on the organ-donation waiting list. Often, they don’t even know about criminal records.
The University of Maryland Medical Center said that “it is the solemn obligation of any hospital or healthcare organisation to provide lifesaving care to every patient who comes through their doors based on their medical needs, not their background or life circumstances”.
David Bennett Jnr had revealed that hospitals refused to add his father to their waiting lists because he previously failed to follow doctors’ orders, take his medication regularly, and attend follow-up visits.
See more from MedicalBrief archives:
First pig-to-human kidney transplant
Gene-edited piglets opening door to animal organ transplants
US opioid epidemic increases availability of organs for donation
How a historic heart transplant created SA's first celebrity scientist 50 years ago